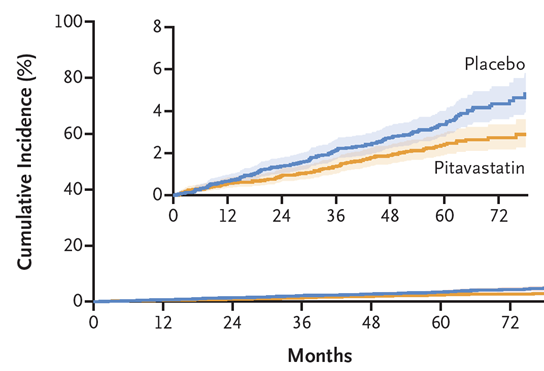
The primary results of REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) were published in the New England Journal of Medicine. In 7,769 people at low-moderate cardiovascular disease risk who were on antiretroviral therapy for HIV, pitavastatin reduced the risk of major adverse cardiovascular events (MACE including myocardial infarction and stroke) by 35% over a median of 5.1 years. This is a landmark result that will have a substantial impact on the treatment and health of many people with HIV.
804 REPRIEVE participants enrolled in the embedded REPRIEVE Mechanistic Substudy, with the aim to assess the effect of pitavastatin on coronary plaque and inflammatory biomarkers. Pitavastatin reduced noncalcified coronary plaque volume by 7% and plaque progression by 33%, as assessed on baseline and 2-year follow-up coronary CT angiography. Pitavastatin also reduced inflammatory biomarkers. These findings may explain the 35% reduction in MACE observed in the parent trial.
This is also the largest randomized controlled trial to apply coronary CT angiography to noninvasively measure changes in coronary plaque in response to a therapy. The substudy was presented at the 2023 American Heart Association Late Breaking Science session.
CIRC is the Data Coordinating Center and CT core laboratory for REPRIEVE. Congratulations to the many CIRC members who made major contributions to the trial over the years.