Over the last few years, the group, together with co-PI Dr. Marielle Scherrer-Crosbie at UPENN, have been working on STOP-CA. Anthracyclines are a commonly used chemotherapeutic. However, the use of anthracyclines is associated with a dose-related cardiac toxicity which typically manifests as a reduction in cardiac function. This study asked whether a year of treatment with atorvastatin, 40 mg/d, started prior to anthracycline-based chemotherapy among patients with lymphoma, reduce the chance of a significant decrease in left ventricular ejection fraction (LVEF) compared with placebo?
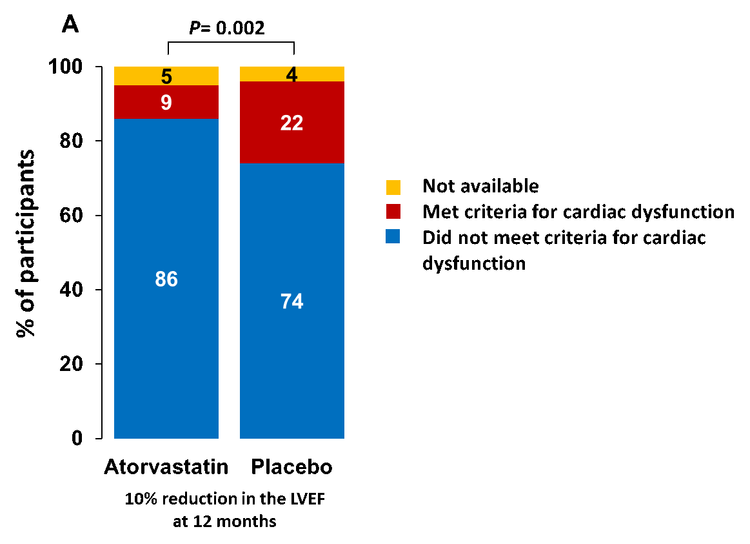
We found, in a randomized trial of 300 adult patients with lymphoma, the percentage of patients with a decrease in cardiac function (>10% absolute decline in LVEF from prior to chemotherapy to a final value of <55% over the 12-month study period), among those randomized to atorvastatin was 9% compared with a rate of 22% among patients randomized to placebo (See figure). This difference met statistical significance. This finding may support the use of atorvastatin in patients with lymphoma at risk of cardiac dysfunction due to anthracycline treatment.